The polymers around us are basically a disordered mess, with long chains of atoms tangled around each other. But starting around 1990, chemists began developing techniques that allow us to build polymers with precisely defined structures. These polymers, called metal-organic frameworks (MOFs), have distinct chemical properties: large pores that can be used to filter or store gases, catalytic centers within the polymer, and more.
On Wednesday, the Nobel Prize Committee honored three researchers for their role in developing MOFs: Richard Robson for demonstrating the first MOF, and Susumu Kitagawa and Omar Yaghi for developing them to their full potential.
Building a structure
Most polymers are built from individual molecules that are linked together by flexible bonds, allowing the molecules to flop around. As a result, in their final form as something like a plastic bag or a bike tire, the polymers are a complicated tangle, with molecules wrapped around each other in no particular order. We can still control some aspects of the resulting polymer by manipulating its bulk properties or changing the molecules it's built from, but there's not a lot of chemistry we can do beyond that.
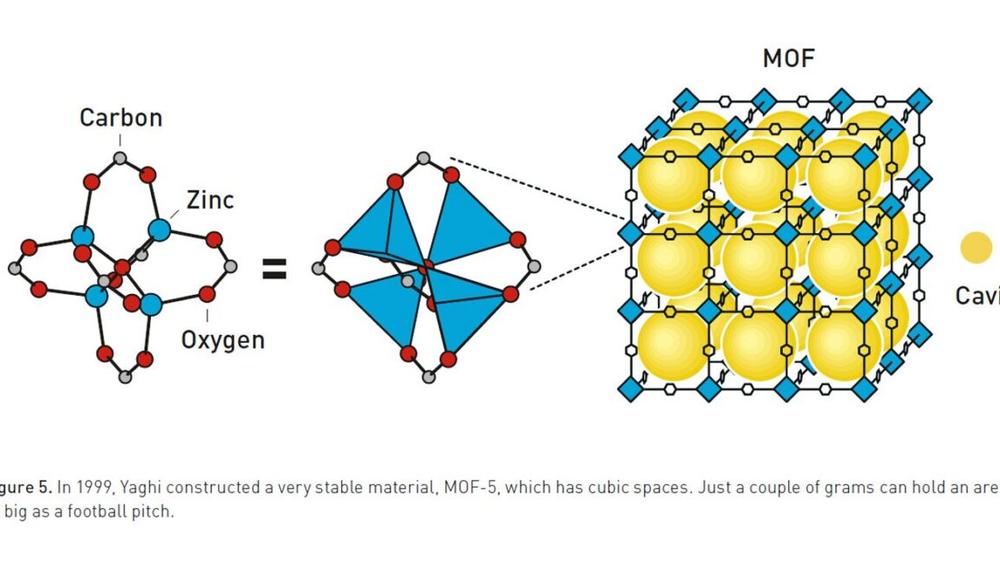
With MOFs, the metal portion of things allows us to control the geometry of how molecules get linked together. The metals act a bit like hubs, with rigid organic molecules extending out in multiple directions. Because of the nature of their orbitals, metals can bond with a specific number of organic molecules, and each of those bonds has set angles between it and all the other bonds. So by choosing a metal carefully, it's possible to craft specific three-dimensional structures, with metals and organic molecules alternating with each other.
Unlike traditional polymers, this structure allows MOFs to have open internal spaces with a well-defined size, which can allow some molecules to pass through while filtering out others. In addition, the presence of metals provides for interesting chemistry. The metals can serve as catalysts or preferentially bind to one molecule within a mixture.
Knowing what we know now, it all seems kind of obvious that this would work. But when Robson started his work at the University of Melbourne, the few people who thought about the issue at all expected that the molecules he was building would be unstable and collapse.
The first MOF Robson built used copper as its metal of choice. It was linked to an organic molecule that retained its rigid structure through the presence of a benzene ring, which doesn't bend. Both the organic molecule and the copper could form four different bonds, allowing the structure to grow by doing the rough equivalent of stacking a bunch of three-sided pyramids—a conscious choice by Robson.
In this case, however, the internal cavities remained filled by the solvent in which the MOF was formed. But the solvent could move freely through the material. Still, based on this example, Robson predicted many of the properties that have since been engineered into different MOFs: the ability to retain their structure even after solvents are removed, the presence of catalytic sites, and the ability of MOFs to act as filters.
Expanding the concept
All of that might seem a very optimistic take for someone's first effort. But the measure of Robson's success is that he convinced other chemists of the potential. One was Susumu Kitagawa of Kyoto University. Kitagawa and his collaborators built a MOF that had large internal channels that extended the entire length of the material. Made in a watery solution, the MOF could be dried out and have gas flow through it, with the structure retaining molecules like oxygen, nitrogen, and methane.
Kitagawa also had some ideas on where the field might be headed, suggesting that it would ultimately be possible to build MOFs that change their properties in response to external conditions, like temperature or illumination, something that has since been demonstrated.
But Yaghi may be the king of crafting new MOF variants. He started out as a Palestinian refugee in Jordan, made his way to the US in his teens, and began his chemistry education at a community college. After making his way to a four-year college and graduate school, Yaghi has since held professorships at four different universities and now holds a university professor position at the University of California, Berkeley. He and his collaborators have built MOFs that are stable at hundreds of degrees Celsius, are roughly 60 percent open space, or could be built with a customizable internal pore size.
Two of Yaghi's notable creations tackle real-world problems. One enables the selective absorption of carbon dioxide, an ability that may come in useful if we want to correct for our excessive greenhouse gas emissions. Another would retain water from desert air overnight and then release it when heated by the Sun the next day.
By now, plenty of different MOFs have been synthesized—their Wikipedia page is now over 20,000 words long—and there has been a steady flow of papers describing new ones with potentially useful features. But this is the sort of development that will probably never capture the public's attention outside of events like this year's Nobel. There are many potential uses for MOFs, but in pretty much all of them, the material will be working behind the scenes to enable something useful but not very glamorous, doing things like storing hydrogen, filtering out carbon dioxide, and catalyzing chemical reactions.
But the range of applications is so large that it's nice to see this year's Nobels give the work some public recognition.
 Microsoft is moving GitHub over to Azure servers
Microsoft is moving GitHub over to Azure servers