For many beer lovers, a nice thick head of foam is one of life's pure pleasures, and the longer that foam lasts, the better the beer-drinking experience. A team of Swiss researchers spent seven years studying why some beer foams last longer than others and found that the degree of fermentation—i.e., whether a given beer has been singly, doubly, or triply fermented—is crucial, according to a new paper published in the journal Physics of Fluids.
As previously reported, foams are ubiquitous in everyday life, found in foods (whipped cream), beverages (beer, cappuccino), shaving cream and hair-styling mousse, packing peanuts, building insulation, flame-retardant materials, and so forth. All foams are the result of air being beaten into a liquid formula that contains some kind of surfactant (active surface agent), usually fats or proteins in edible foams, or chemical additives in non-edible products. That surfactant strengthens the liquid film walls of the bubbles to keep them from collapsing.
Individual bubbles typically form a sphere because that's the shape with the minimum surface area for any volume and hence is the most energy-efficient. One reason for the minimizing principle when it comes to a bubble's shape is that many bubbles can then tightly pack together to form a foam. But bubbles "coarsen" over time, the result of gravity pulling down on the liquid and thinning out the walls. Eventually, they start to look more like soccer balls (polyhedrons). In a coarsening foam, smaller bubbles are gradually absorbed by larger ones. There is less and less liquid to separate the individual bubbles, so they press together to fill the space.
This "jamming" is why foams are typically far more rigid than their gas (95 percent) and liquid (5 percent) components. The more tightly the bubbles jam together, the less they can move around and the greater the pressure inside them becomes, giving them properties of a solid.
Various factors can affect foam stability. For instance, in 2019, Japanese researchers investigated a phenomenon known as "collective bubble collapse," or CBC, in which breaking one bubble at the edge of a foam results in a cascading effect as the breakage spreads to other bubbles in the foam. They identified two distinct mechanisms for the resulting CBCs: a so-called "propagating mode," in which a broken bubble is absorbed into the liquid film, and a "penetrating mode," in which the breakage of a bubble causes droplets to shoot off and hit other bubbles, causing them to break in turn.
Higher levels of liquid in the foam slowed the spread of the collapse, and changing the viscosity of the fluid had no significant impact on how many bubbles broke in the CBC. Many industrial strategies for stabilizing foams rely on altering the viscosity; this shows those methods are ineffective. The researchers suggest focusing instead on using several different surfactants in the mixture. This would strengthen the resulting film to make it more resistant to breakage when hit by flying droplets.
However, as the authors of this latest paper note, "Most beers are not detergent solutions (though arguably some may taste that way)." They were inspired by a Belgian brewer's answer when they asked how he controlled fermentation: "By watching the foam." A stable foam is considered to be a sign of successful fermentation. The authors decided to investigate precisely how the various factors governing foam stability might be influenced by the fermentation process.
Triple that fermentation
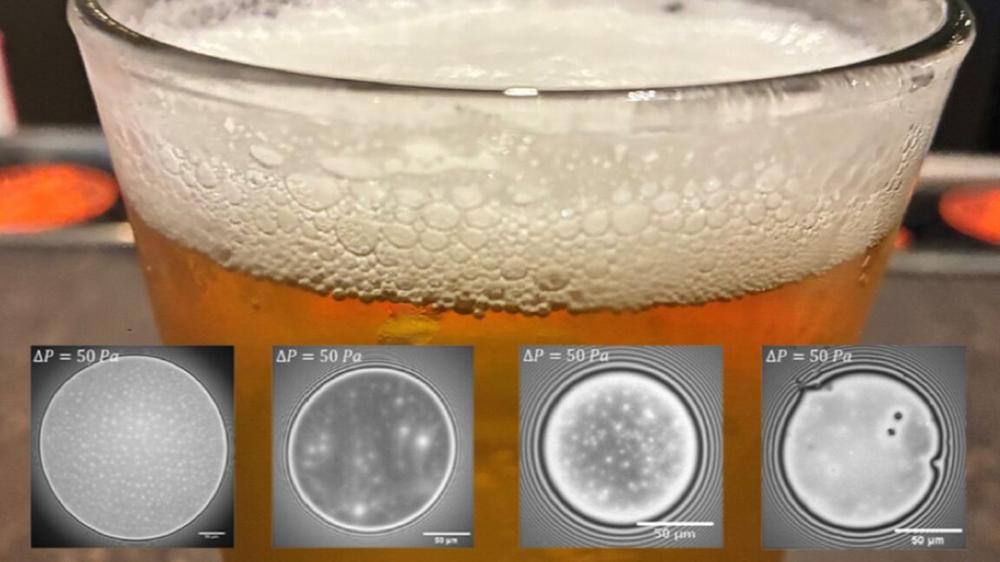
“The idea was to directly study what happens in the thin film that separates two neighboring bubbles,” said co-author Emmanouil Chatzigiannakis of ETH Zurich in Switzerland and Eindhoven University of Technology in the Netherlands. “And the first thing that comes to mind when thinking of bubbles and foams is beer.”
To that end, Chatzigiannakis et al. conducted experiments on six commercial beers: two triple-fermented Belgian beers (Westmalle Tripel and Tripel Karmeliet); two Swiss lagers (Feldschlösschen and Chopfab); and two additional Belgian beers: the single-fermented Westmalle Extra and the double-fermented Westmalle Dubbel.
Single-fermented lager beers had the least stable foam, with triple-fermented beers boasting the most stable foam; the foam stability of double-fermented beers fell in the middle of the range. The team also discovered that the most important factor for foam stability isn't fixed but largely depends on the type of beer. It all comes down to surface viscosity for single-fermented lagers.
But surface viscosity is not a major factor for stable foams in double- or triple-fermented beers. Instead, stability arises from differences in surface tension, i.e., Marangoni stresses—the same phenomenon behind so-called "wine tears" and the "coffee ring effect." Similarly, when a drop of watercolor paint dries, the pigment particles of color break outward toward the rim of the drop. In the case of beer foam, the persistent currents that form as a result of those differences in surface tension lend stability to the foam.
The researchers also analyzed the protein content of the beers and found that one in particular—lipid transfer protein 1 (LPT1)—was a significant factor in stabilizing beer foams, and their form depended on the degree of fermentation. In single-fermented beers, for example, the proteins are small, round particles on the surface of the bubbles. The more proteins there are, the more the foam will be stable because those proteins form a more viscous film around the bubbles.
Those LPT1 proteins become slightly denatured during a second fermentation, forming more of a net-like structure that improves foam stability. That denaturation continues during a third fermentation, when the proteins break down into fragments with hydrophobic and hydrophilic ends, reducing surface tensions. They essentially become surfactants to make the bubbles in the foam much more stable.
That said, the team was a bit surprised to find that increasing the viscosity with additional surfactants can actually make the foam more unstable because it slows down the Marangoni effects too strongly. "The stability of the foam does not depend on individual factors linearly," said co-author Jan Vermant, also of ETH Zurich. "You can't just change 'something' and get it 'right.' The key is to work on one mechanism at a time–and not on several at once. Beer obviously does this well by nature. We now know the mechanism exactly and are able to help [breweries] improve the foam of their beers.”
The findings likely have broader applications as well. “This is an inspiration for other types of materials design, where we can start thinking about the most material-efficient ways [of creating stable foams],” said Vermant. “If we can't use classical surfactants, can we mimic the 2D networks that double-fermented beers have?” The group is now investigating how to prevent lubricants used in electric vehicles from foaming; developing sustainable surfactants that don't contain fluoride or silicon; and finding ways to use proteins to stabilize milk foam, among other projects.
DOI: Physics of Fluids, 2025. 10.1063/5.0274943 (About DOIs).
 Gears of War: Reloaded Review Update
Gears of War: Reloaded Review Update