“Illusions are fun, but they are also a gateway to perception,” says Hyeyoung Shin, assistant professor of neuroscience at Seoul National University. Shin is the first author of a new study in Nature Neuroscience that has identified a specific population of neurons in the visual cortex—dubbed IC-encoders—and shows their direct role in representing a visual illusion. The work is the result of a collaboration between the University of California, Berkeley, the Allen Institute in Seattle, and Seoul National University.
What the brain “knows”
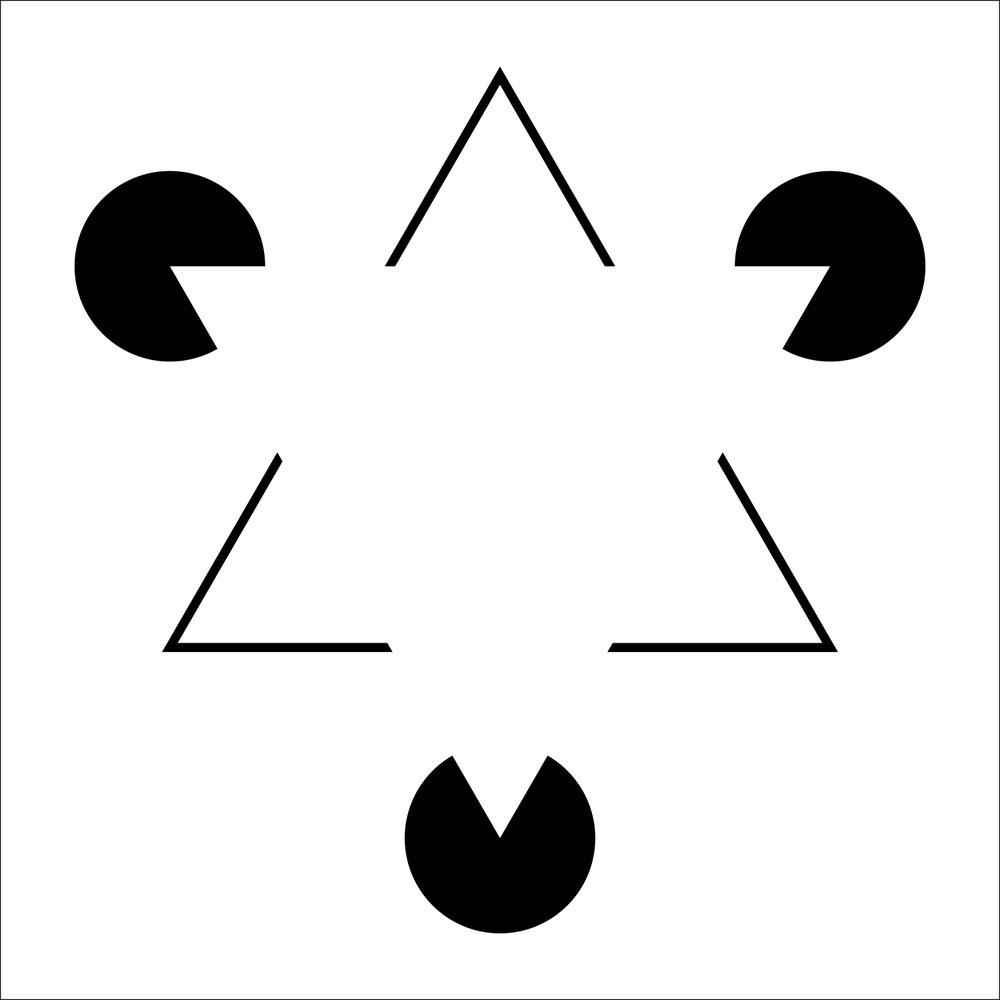
Illusory contours are edges we see even though they aren’t physically there. A classic example is the Kanizsa triangle: three “Pac-Man” shapes make us perceive a bright white triangle floating on top. Hide the Pac-Men with your fingers and the trick is revealed: There is no border, just a uniform background. Neurophysiology agrees with perception here: For over 20 years, studies in primates and later imaging in humans and mice have described neurons in the primary visual cortex (V1) and higher visual areas that respond to real and illusory contours.
If the edges don’t exist in the stimulus, the brain must be filling them in. That’s tricky to explain in a purely “bottom-up” view of perception, where the visual system would just act like a camera, faithfully processing the input from the retina. But even at the very first stages, the visual system doesn’t just passively record—it integrates local and long-range neural signals and draws on prior knowledge (like statistical regularities of the visual world) to guide its interpretations. In other words, it makes “assumptions” about what we’re seeing based on what it already knows.
The new study pinpoints the neurons that encode some of these inferences. To find them, the researchers combined cutting-edge brain imaging with a crucial causal test: directly poking the neurons to see what happened.
Watching and poking neurons
The first part of the study, done with the Allen Institute’s OpenScope program, used high-density silicon probes to record large-scale electrical activity across visual areas of mouse brains, capturing the activity of hundreds of neurons with sub-millisecond precision as the mice viewed stimuli that included illusory contours.
That technique has superb temporal resolution, but it can’t easily distinguish the properties of individual cells. So the team also recorded the activity of thousands of neurons in the upper layers of the visual cortex using a calcium-sensitive fluorescent molecule. This produced what Hillel Adesnik of UC Berkeley calls “dynamic pictures of the brain activity where neurons flash when they fire.”
Together, these approaches not only mapped activity but also identified the specific neurons to target in the next phase with optogenetics. This technique introduces the gene encoding a light-sensitive protein into the genomes of neurons, allowing light to act like an on/off switch. Shine light, and neurons fire. Adesnik’s group took this a step further, using holographic 3D illumination: “We used an optical system that shapes the light in three dimensions that allowed us to target very specific neurons,” he says.
This allowed them to stimulate a tiny, very selective subset: “Out of approximately 5,000 neurons, we were able to selectively target the 20 most responsive to, for example, encoding illusory contours.” By combining this stimulation with cortical recordings, the team could move beyond simply correlating neural activity with a stimulus. As Adesnik puts it: “rather than working with a purely observational approach, we could actually photo-stimulate and activate those particular neurons as a group and observe how that influences neural dynamics.”
By photo-stimulating the IC-encoders, the researchers were able to re-create the same neural activity patterns normally evoked by illusory edges, even in the absence of any visual input. In other words, activating this population was enough to generate the specific ‘border’ signal in the visual circuit, not just a generic burst of activity. This suggests IC-encoders don’t just follow sensory input—they actively build the representation of edges that aren’t physically there.
Earlier work had hinted at such cells, but Shin and colleagues show systematically that they’re not rare oddballs—they’re a well-defined, functionally important subpopulation. “What we didn’t know is that these neurons drive local pattern completion within primary visual cortex,” says Shin. “We showed that those cells are causally involved in this pattern completion process that we speculate is likely involved in the perceptual process of illusory contours,” adds Adesnik.
Behavioral tests still to come
That doesn’t mean the mice “saw” the illusory contours when the neurons were artificially activated. “We didn’t actually measure behavior in this study,” says Adesnik. “It was about the neural representation.” All we can say at this point is that the IC-encoders could induce neural activity patterns that matched what imaging shows during normal perception of illusory contours.
“It’s possible that the mice weren’t seeing them,” admits Shin, “because the technique has involved a relatively small number of neurons, for technical limitations. But in the future, one could expand the number of neurons and also introduce behavioral tests.”
That’s the next frontier, Adesnik says: “What we would do is photo-stimulate these neurons and see if we can generate an animal’s behavioral response even without any stimulus on the screen.” Right now, optogenetics can only drive a small number of neurons, and IC-encoders are relatively rare and scattered. “For now, we have only stimulated a small number of these detectors, mainly because of technical limitations. IC-encoders are a rare population, probably distributed through the layers [of the visual system], but we could imagine an experiment where we recruit three, four, five, maybe even 10 times as many neurons,” he says. “In this case, I think we might be able to start getting behavioral responses. We’d definitely very much like to do this test.”
Nature Neuroscience, 2025. DOI: 10.1038/s41593-025-02055-5
Federica Sgorbissa is a science journalist; she writes about neuroscience and cognitive science for Italian and international outlets.
 Pizza restaurant responds after finding out Dave Portnoy lied about review score
Pizza restaurant responds after finding out Dave Portnoy lied about review score